Currently viewing
Menu
Crevice Corrosion - localized corrosion of a metal or alloy surface at, or immediately adjacent to, an area that is shielded from full exposure to the environment because of close proximity of the metal or alloy to the surface of another material or an adjacent surface of the same metal or alloy. [1]
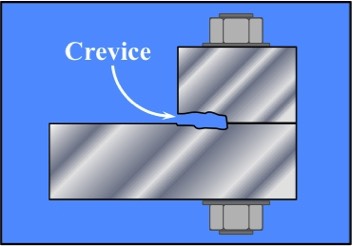
Schematic of Crevice Corrosion
Between Two Bolted Plates
Crevice Corrosion is enhanced corrosion in "occluded" (tight-fitting closed) regions between mating surfaces that are not moving. The mechanism of Crevice Corrosion involves "crevice deaeration" (lowering of oxygen) - followed by the development of a more acidic solution in the crevice which exacerbates the corrosion reaction.
Corrosion in an aqueous solution of sodium chloride is initially distributed uniformly over the entire surface of the metal, including the crevice. After a short period of time, the oxygen in the crevice becomes depleted. This happens when the oxygen in the crevice is depleted by the Oxygen Reduction Reaction (ORR) - in combination with the slow replenishment of oxygen in the crevice due to the slow transport properties of oxygen. The crevice is then said to be deaerated. The ORR becomes localized outside the crevice. Corrosion continues inside the crevice and, as a result, there is a build up of metal ions. This build up of positive charge inside the crevice is counteracted when negatively charged chloride ions migrate into the crevice. Metal chlorides inside the crevice react with water, and the result is metal hydroxides and acid. Acidification of the crevice exacerbates corrosion.
Although compositional changes within the crevice are certainly part of the mechanism, they are not the only factor. There is also a contribution from IR Drop (or Ohmic Drop). The difference in potential can result in an alloy that is active in the crevice, but passive on the exposed surface - that is to say: for an active-passive alloy the potential within the crevice can be below the Primary Passivation Potential (Epp). At the same time, the potential of the exposed surface can be above Epp and within the passive region for that material.
The most effective control for Crevice Corrosion is to avoid geometries conducive to crevice formation. The possibility of deposits should also be avoided, as they can create an occluded region. If it is not possible to avoid a crevice, sealing (caulking, welding, etc.) may help.
Filiform Corrosion - corrosion that occurs under some coatings in the form of randomly distributed threadlike filaments. [1]
Filiform Corrosion is an unusual form of Crevice Corrosion that can develop under thin films. It has been observed beneath both polymer and metal thin films, and takes the form of filaments. While this type of corrosion causes little damage to the metal, it spoils the appearance of the surface. So - this form of corrosion is especially detrimental when it develops on food packaging.
Corrosion in an aqueous solution of sodium chloride is initially distributed uniformly over the entire surface of the metal, including the crevice. After a short period of time, the oxygen in the crevice becomes depleted. This happens when the oxygen in the crevice is depleted by the Oxygen Reduction Reaction (ORR) - in combination with the slow replenishment of oxygen in the crevice due to the slow transport properties of oxygen. The crevice is then said to be deaerated. The ORR becomes localized outside the crevice. Corrosion continues inside the crevice and, as a result, there is a build up of metal ions. This build up of positive charge inside the crevice is counteracted when negatively charged chloride ions migrate into the crevice. Metal chlorides inside the crevice react with water, and the result is metal hydroxides and acid. Acidification of the crevice exacerbates corrosion.
Although compositional changes within the crevice are certainly part of the mechanism, they are not the only factor. There is also a contribution from IR Drop (or Ohmic Drop). The difference in potential can result in an alloy that is active in the crevice, but passive on the exposed surface - that is to say: for an active-passive alloy the potential within the crevice can be below the Primary Passivation Potential (Epp). At the same time, the potential of the exposed surface can be above Epp and within the passive region for that material.
The most effective control for Crevice Corrosion is to avoid geometries conducive to crevice formation. The possibility of deposits should also be avoided, as they can create an occluded region. If it is not possible to avoid a crevice, sealing (caulking, welding, etc.) may help.
Filiform Corrosion - corrosion that occurs under some coatings in the form of randomly distributed threadlike filaments. [1]
Filiform Corrosion is an unusual form of Crevice Corrosion that can develop under thin films. It has been observed beneath both polymer and metal thin films, and takes the form of filaments. While this type of corrosion causes little damage to the metal, it spoils the appearance of the surface. So - this form of corrosion is especially detrimental when it develops on food packaging.
[1] NACE/ASTM G193-10b Standard Terminology and Acronyms Relating to Corrosion, 2010. All rights reserved by NACE. (Reprinted with Permission)
Currently viewing
Page last updated: 12/16/23
