Currently viewing
Menu
Alternating Current (AC) Tests - Polymer-Coated Metals
One method for reducing corrosion damage is painting or coating with an organic - and one of the most widely employed techniques for evaluating such polymer-coated systems is EIS (Electrochemical Impedance Spectroscopy).
While degradation depends on a number of variables, coatings isolate the metallic substrate from direct contact with the environment and delay the arrival of reactants at the interface.
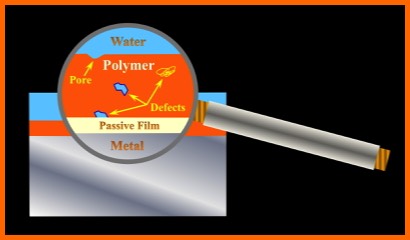
However, as presented in the Figure below, coatings tend to contain pores and virtual pores (regions of poor cross-linking), as well as both internal and interfacial defects. As a result, Underfilm Corrosion is still likely to develop in time.
While degradation depends on a number of variables, coatings isolate the metallic substrate from direct contact with the environment and delay the arrival of reactants at the interface.
However, as presented in the Figure below, coatings tend to contain pores and virtual pores (regions of poor cross-linking), as well as both internal and interfacial defects. As a result, Underfilm Corrosion is still likely to develop in time.
When a metal corrodes, it experiences a change in mass as a function of exposure time. Mass Loss is, in many respects, the reference measurement against which all other techniques are assessed. While Lorenz and Mansfeld [Corros. Sci., Vol. 21, p. 647 (1981)] demonstrated the association between EIS data and the corrosion rate for bare metals in 1981, the analogous relationship for polymer-coated metals was not accurately defined until almost 20 years later.
Mass loss for bare samples can easily be defined using a traditional gravimetric approach. Samples are weighed before and after a test - and the mass loss is the difference between the initial and final weight.
The use of a traditional gravimetric approach (such as mass loss) is not possible for polymer-coated samples. The difficulty in finding the mass loss for polymer-coated samples is associated with solution uptake within the polymer, as well as corrosion product entrapment at the coating/metal interface. Both solution uptake and corrosion product entrapment can change the apparent mass of a sample. These issues were overcome during our research described in the the two papers referenced below. During this study, the metallic mass loss for polymer-coated samples was assessed using a magnetic measurement technique - and then correlated to equivalent data produced by EIS.
Mass loss for bare samples can easily be defined using a traditional gravimetric approach. Samples are weighed before and after a test - and the mass loss is the difference between the initial and final weight.
The use of a traditional gravimetric approach (such as mass loss) is not possible for polymer-coated samples. The difficulty in finding the mass loss for polymer-coated samples is associated with solution uptake within the polymer, as well as corrosion product entrapment at the coating/metal interface. Both solution uptake and corrosion product entrapment can change the apparent mass of a sample. These issues were overcome during our research described in the the two papers referenced below. During this study, the metallic mass loss for polymer-coated samples was assessed using a magnetic measurement technique - and then correlated to equivalent data produced by EIS.
EIS is currently employed to rank coatings, assess interfacial reactions, quantify coating breakdown, and predict the lifetime of coating/metal systems.
Equivalent Circuits are frequently used by researchers to represent the particular system that is being assessed. An equivalent circuit must exhibit a correlation with the physical system it represents.
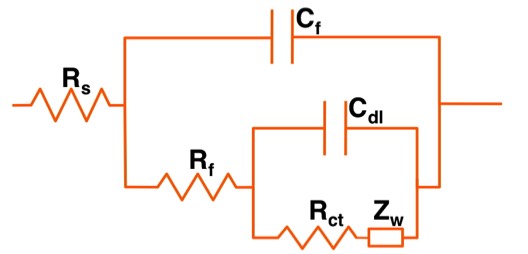
The most common equivalent circuit employed for polymer-coated systems is shown in the Figure below.
Equivalent Circuits are frequently used by researchers to represent the particular system that is being assessed. An equivalent circuit must exhibit a correlation with the physical system it represents.
The most common equivalent circuit employed for polymer-coated systems is shown in the Figure below.
Rs = Electrolyte Resistance
Rf = Coating Resistance
Cf = Coating Capacitance
Rct = Charge-Transfer Resistance
Cdl = Double-Layer Capacitance
Zw = Warburg Impedance (Diffusion)
Rs = Electrolyte Resistance
Rf = Coating Resistance
Cf = Coating Capacitance
Rct = Charge-Transfer Resistance
Cdl = Double-Layer Capacitance
Zw = Warburg Impedance (Diffusion)

Rs = Electrolyte Resistance
Rf = Coating Resistance
Cf = Coating Capacitance
Rct = Charge-Transfer Resistance
Cdl = Double-Layer Capacitance
Zw = Warburg Impedance (Diffusion)

For an intact (non-degraded) polymer-coated metal system, only the external circuit (Rs, Rf, Cf) will be observed experimentally. With time, however, the embedded circuit (Rct, Cdl, Zw) may also become active, and this is the source of a second time constant. This second time constant has been associated with system breakdown.
"In Situ Underfilm Corrosion Rate Measurement by Magnetic and Electrochemical Techniques", Electrochemical and Solid-State Letters, Vol. 3, p. 275 (2000).
"The Correlation Between Substrate Mass Loss and Electrochemical Impedance Spectroscopy Data for a Polymer-Coated Metal", J. Electrochem. Soc., Vol. 149, p. B265 (2002).
"The Correlation Between Substrate Mass Loss and Electrochemical Impedance Spectroscopy Data for a Polymer-Coated Metal", J. Electrochem. Soc., Vol. 149, p. B265 (2002).
Currently viewing
Page last updated: 12/16/23
