Currently viewing
Menu
We can use Faraday's Law to find the Mass Loss during an electrochemical experiment.
Faraday's Law shows that the Mass Loss (Δm) is equal to the Current (I) times the Time (t) times the Atomic Mass (a) divided by the Valence Change (n) and Faraday's Constant (F).
Faraday's Law shows that the Mass Loss (Δm) is equal to the Current (I) times the Time (t) times the Atomic Mass (a) divided by the Valence Change (n) and Faraday's Constant (F).
Faraday's Law
Δm = Ita/nF
In the equation below, the Mass Loss (Δm) per Unit Area (A) per Unit Time (t) is equal to the Corrosion Rate (CR).
CR = Δm/At
If we substitute Faraday's Law for Mass Loss (Δm), we obtain the equation below:
CR = Ita/nFAt
Time (t) on the top and bottom of the above equation cancel each other out. As a result, the equation is reduced to:
CR = Ia/nFA
The Current (I) divided by the Area (A) is equal to the Current Density (i). When we replace I/A (in the above equation) with i, the new equation becomes:
CR = ia/nF
The Current Density (i) in the above equation is the same as the icorr that is found during an electrochemical experiment - so, the final equation is:
CR = icorra/nF
We already know the Atomic Mass (a), the Valence Change (n), and Faraday's Constant (F). If the Corrosion Current Density (icorr) can be defined during a DC electrochemical experiment - such as the Schematic of the Tafel Extrapolation Test shown below - the Corrosion Rate can then be calculated.
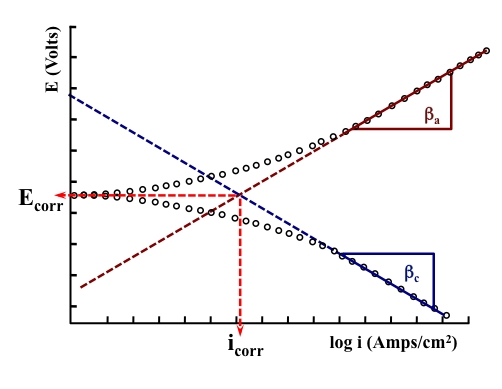
A Penetration Rate (such as mmpy or mpy) can be found by dividing the above equation with ρ (density). The new equation then becomes:
CR = icorra/nFρ
Currently viewing
Page last updated: 12/16/23
