Currently viewing
Menu
Dealloying
Dealloying - a corrosion process whereby one constituent of an alloy is preferentially removed, leaving an altered residual structure. [also known as parting, selective dissolution, or selective leaching] [1]
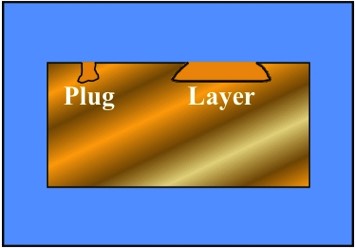
Schematic Representation Showing
Plug-Type and Layer-Type Dealloying
There is frequently little difference in the appearance of a metal or alloy during Dealloying - except for a possible change in color. Nevertheless, the properties of the alloy may have changed substantially. Plug-Type Dealloying involves the local loss of the anodic component of the alloy, and Layer-Type Dealloying involves more uniform attack.
Dezincification is a well-known type of Dealloying during which Zinc (Zn) is lost from Brass (Cu-Zn). Two mechanisms have been proposed for Dezincification: The first mechanism suggests that the Zinc (Zn) is leached out of the alloy. The second mechanism proposes that the entire alloy dissolves, but the Copper (Cu) is redeposited on the surface. Both have scientific and experimental support.
In general, Dealloying will only occur if the proportion of the alloy components are correct. In the case of brass, dezincification only occurs if the zinc component is higher than 15%. Red Brass (Cu-15Zn) - for example - suffers minimal dezincification, Alpha Brass (Cu-30Zn) is highly susceptible to dezincification, and Beta Brass (Cu-40Zn) is even more susceptible.
Copper Zinc Alloys are not the only alloys that are subject to Dealloying. Cast Iron can suffer from the dealloying of iron, leaving graphite behind. We have also reported seeing dealloying in High-Nickel Alloys exposed to acidic Supercritical Water Oxidation (SCWO) systems at elevated temperatures.
Dezincification is a well-known type of Dealloying during which Zinc (Zn) is lost from Brass (Cu-Zn). Two mechanisms have been proposed for Dezincification: The first mechanism suggests that the Zinc (Zn) is leached out of the alloy. The second mechanism proposes that the entire alloy dissolves, but the Copper (Cu) is redeposited on the surface. Both have scientific and experimental support.
In general, Dealloying will only occur if the proportion of the alloy components are correct. In the case of brass, dezincification only occurs if the zinc component is higher than 15%. Red Brass (Cu-15Zn) - for example - suffers minimal dezincification, Alpha Brass (Cu-30Zn) is highly susceptible to dezincification, and Beta Brass (Cu-40Zn) is even more susceptible.
Copper Zinc Alloys are not the only alloys that are subject to Dealloying. Cast Iron can suffer from the dealloying of iron, leaving graphite behind. We have also reported seeing dealloying in High-Nickel Alloys exposed to acidic Supercritical Water Oxidation (SCWO) systems at elevated temperatures.
[1] NACE/ASTM G193-10b Standard Terminology and Acronyms Relating to Corrosion, 2010. All rights reserved by NACE. (Reprinted with Permission)
Currently viewing
Page last updated: 3/4/25
