Currently viewing
Menu
Unit 7 - Electrochemical Nature of Corrosion
FULL SCREEN
Aqueous Corrosion (corrosion in water media) is electrochemical in nature. This means that the corrosion reaction involves electrons (e-).
All aqueous corrosion can be divided into Anodic and Cathodic reactions.
For example, if Zinc (Zn) is placed into Hydrochloric Acid (HCl) - Zinc Chloride (ZnCl2) is formed, along with Hydrogen Gas (H2). This is shown in the following reaction:
Zn + 2HCl → ZnCl2 + H2
The above reaction can also be written as:
Zn + 2H+ + 2Cl- → Zn2+ + 2Cl- + H2
Please note that 2Cl- is the same on both sides in the above reaction. As a result, it can be removed to give the following reaction:
Zn + 2H+ → Zn2+ + H2
The reaction above can be divided into an Anodic Reaction and a Cathodic Reaction:
Zn → Zn2+ + 2e-
2H+ + 2e- → H2
All aqueous corrosion can be divided into Anodic and Cathodic reactions.
For example, if Zinc (Zn) is placed into Hydrochloric Acid (HCl) - Zinc Chloride (ZnCl2) is formed, along with Hydrogen Gas (H2). This is shown in the following reaction:
Zn + 2HCl → ZnCl2 + H2
The above reaction can also be written as:
Zn + 2H+ + 2Cl- → Zn2+ + 2Cl- + H2
Please note that 2Cl- is the same on both sides in the above reaction. As a result, it can be removed to give the following reaction:
Zn + 2H+ → Zn2+ + H2
The reaction above can be divided into an Anodic Reaction and a Cathodic Reaction:
Zn → Zn2+ + 2e-
2H+ + 2e- → H2
An Anodic Reaction is an oxidation process through which the valence of a metal increases from zero to a more positive value.
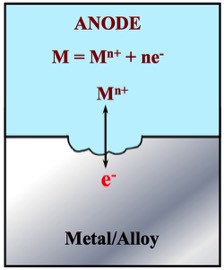
Aqueous Corrosion involves the loss of metal to the aqueous phase. When this occurs, the metal releases a number of electrons (e-) into the Metal/Alloy.
There are many anodic reactions; however, we can identify two main types as follows:
M → Mn+ + ne- In this reaction, Mn+ is a soluble corrosion product that does not protect the surface of the metal.
M + nH2O → M(OH)n + nH+ + ne- In this reaction, M(OH)n is a solid corrosion product that may - or may not - slow corrosion of the metal.
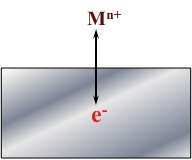
The Figure presented below is a schematic representation of a generic corrosion reaction.
If generic metal M corrodes, it goes into solution as Mn+ and leaves a number (n) of electrons (e-) in the metal.
M → Mn+ + ne-
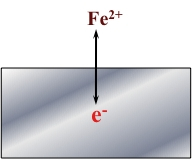
The Figure presented below is a schematic representation of an anodic reaction for Iron (Fe).
In the case of iron, the metal (Fe) goes into solution as the ion Fe2+ and leaves two electrons (e-) in the metal.
Fe → Fe2+ + 2e-
A Cathodic Reaction is a reduction process during which the valence of the species is reduced.
The electrons (e-) produced during the anodic corrosion reaction must be consumed at the same rate by a corresponding cathodic reaction.
While there are only two cathodic reactions in natural environments, there are many more cathodic reactions in various man-made environments.
Cathodic reactions consume electrons (e-) and are reduction reactions. In a reduction reaction, the valence is "reduced" to a lower value.
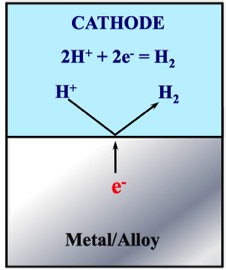
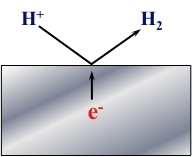
The Figure presented below is a schematic representation of the Hydrogen Evolution Reaction (HER).
In this reaction, 2H+ accept electrons to become Hydrogen Gas (H2).
2H+ + 2e- → H2
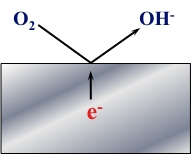
The Figure presented below is a schematic representation of the Oxygen Reduction Reaction (ORR).
In this reaction, Oxygen (O2) in water is reduced to Hydroxyl Ions (OH-).
O2 + 2H2O + 4e- → 4OH-
iPAD
Aqueous Corrosion (corrosion in water media) is electrochemical in nature. This means that the corrosion reaction involves electrons (e-).
All aqueous corrosion can be divided into Anodic and Cathodic reactions.
For example, if Zinc (Zn) is placed into Hydrochloric Acid (HCl) - Zinc Chloride (ZnCl2) is formed, along with Hydrogen Gas (H2). This is shown in the following reaction:
Zn + 2HCl → ZnCl2 + H2
The above reaction can also be written as:
Zn + 2H+ + 2Cl- → Zn2+ + 2Cl- + H2
Please note that 2Cl- is the same on both sides in the above reaction. As a result, it can be removed to give the following reaction:
Zn + 2H+ → Zn2+ + H2
The reaction above can be divided into an Anodic Reaction and a Cathodic Reaction:
Zn → Zn2+ + 2e-
2H+ + 2e- → H2
All aqueous corrosion can be divided into Anodic and Cathodic reactions.
For example, if Zinc (Zn) is placed into Hydrochloric Acid (HCl) - Zinc Chloride (ZnCl2) is formed, along with Hydrogen Gas (H2). This is shown in the following reaction:
Zn + 2HCl → ZnCl2 + H2
The above reaction can also be written as:
Zn + 2H+ + 2Cl- → Zn2+ + 2Cl- + H2
Please note that 2Cl- is the same on both sides in the above reaction. As a result, it can be removed to give the following reaction:
Zn + 2H+ → Zn2+ + H2
The reaction above can be divided into an Anodic Reaction and a Cathodic Reaction:
Zn → Zn2+ + 2e-
2H+ + 2e- → H2
An Anodic Reaction is an oxidation process through which the valence of a metal increases from zero to a more positive value.

Aqueous Corrosion involves the loss of metal to the aqueous phase. When this occurs, the metal releases a number of electrons (e-) into the Metal/Alloy.
There are many anodic reactions; however, we can identify two main types as follows:
M → Mn+ + ne- In this reaction, Mn+ is a soluble corrosion product that does not protect the surface of the metal.
M + nH2O → M(OH)n + nH+ + ne- In this reaction, M(OH)n is a solid corrosion product that may - or may not - slow corrosion.
The Figure presented below is a schematic representation of a generic corrosion reaction.
If generic metal M corrodes, it goes into solution as Mn+ and leaves a number (n) of electrons (e-) in the metal.
M → Mn+ + ne-
The Figure presented below is a schematic representation of an anodic reaction for Iron (Fe).
In the case of iron, the metal (Fe) goes into solution as the ion Fe2+ and leaves two electrons (e-) in the metal.
Fe → Fe2+ + 2e-


A Cathodic Reaction is a reduction process during which the valence of the species is reduced.

The electrons (e-) produced during the anodic corrosion reaction must be consumed at the same rate by a corresponding cathodic reaction.
While there are only two cathodic reactions in natural environments, there are many more cathodic reactions in various man-made environments.
Cathodic reactions consume electrons (e-) and are reduction reactions. In a reduction reaction, the valence is "reduced" to a lower value.
The Figure presented below is a schematic representation of the Hydrogen Evolution Reaction (HER).
In this reaction, 2H+ accept electrons to become Hydrogen Gas (H2).
2H+ + 2e- → H2

The Figure presented below is a schematic representation of the Oxygen Reduction Reaction (ORR).
In this reaction, Oxygen (O2) in water is reduced to Hydroxyl Ions (OH-).
O2 + 2H2O + 4e- → 4OH-

iPHONE
Aqueous Corrosion (corrosion in water media) is electrochemical in nature. This means that the corrosion reaction involves electrons (e-).
All aqueous corrosion can be divided into Anodic and Cathodic reactions.
For example, if Zinc (Zn) is placed into Hydrochloric Acid (HCl) - Zinc Chloride (ZnCl2) is formed, along with Hydrogen Gas (H2). This is shown in the following reaction:
Zn + 2HCl → ZnCl2 + H2
The above reaction can also be written as:
Zn + 2H+ + 2Cl- → Zn2+ + 2Cl- + H2
Please note that 2Cl- is the same on both sides in the above reaction. As a result, it can be removed to give the following reaction:
Zn + 2H+ → Zn2+ + H2
The reaction above can be divided into an Anodic Reaction and a Cathodic Reaction:
Zn → Zn2+ + 2e-
2H+ + 2e- → H2
All aqueous corrosion can be divided into Anodic and Cathodic reactions.
For example, if Zinc (Zn) is placed into Hydrochloric Acid (HCl) - Zinc Chloride (ZnCl2) is formed, along with Hydrogen Gas (H2). This is shown in the following reaction:
Zn + 2HCl → ZnCl2 + H2
The above reaction can also be written as:
Zn + 2H+ + 2Cl- → Zn2+ + 2Cl- + H2
Please note that 2Cl- is the same on both sides in the above reaction. As a result, it can be removed to give the following reaction:
Zn + 2H+ → Zn2+ + H2
The reaction above can be divided into an Anodic Reaction and a Cathodic Reaction:
Zn → Zn2+ + 2e-
2H+ + 2e- → H2
An Anodic Reaction is an oxidation process through which the valence of a metal increases from zero to a more positive value.
Aqueous Corrosion involves the loss of metal to the aqueous phase. When this occurs, the metal releases a number of electrons (e-) into the Metal/Alloy.
There are many anodic reactions; however, we can identify two main types as follows:
M → Mn+ + ne- In this reaction, Mn+ is a soluble corrosion product that does not protect the surface of the metal.
M + nH2O → M(OH)n + nH+ + ne- In this reaction, M(OH)n is a solid corrosion product that may - or may not - slow corrosion of the metal.
A Cathodic Reaction is a reduction process during which the valence of the species is reduced.
The electrons (e-) produced during the anodic corrosion reaction must be consumed at the same rate by a corresponding cathodic reaction.
While there are only two cathodic reactions in natural environments, there are many more cathodic reactions in various man-made environments.
Cathodic reactions consume electrons (e-) and are reduction reactions. In a reduction reaction, the valence is "reduced" to a lower value.
One Cathodic reaction is the Hydrogen Evolution Reaction (HER). In this reaction, 2H+ accept electrons (e-) to become Hydrogen Gas (H2).
2H+ + 2e- → H2
Another Cathodic reaction is the Oxygen Reduction Reaction (ORR).
In this reaction, Oxygen (O2) in water is reduced to Hydroxyl Ions (OH-).
O2 + 2H2O + 4e- → 4OH-
Aqueous Corrosion involves the loss of metal to the aqueous phase. When this occurs, the metal releases a number of electrons (e-) into the Metal/Alloy.
There are many anodic reactions; however, we can identify two main types as follows:
M → Mn+ + ne- In this reaction, Mn+ is a soluble corrosion product that does not protect the surface of the metal.
M + nH2O → M(OH)n + nH+ + ne- In this reaction, M(OH)n is a solid corrosion product that may - or may not - slow corrosion of the metal.
A Cathodic Reaction is a reduction process during which the valence of the species is reduced.
The electrons (e-) produced during the anodic corrosion reaction must be consumed at the same rate by a corresponding cathodic reaction.
While there are only two cathodic reactions in natural environments, there are many more cathodic reactions in various man-made environments.
Cathodic reactions consume electrons (e-) and are reduction reactions. In a reduction reaction, the valence is "reduced" to a lower value.
One Cathodic reaction is the Hydrogen Evolution Reaction (HER). In this reaction, 2H+ accept electrons (e-) to become Hydrogen Gas (H2).
2H+ + 2e- → H2
Another Cathodic reaction is the Oxygen Reduction Reaction (ORR).
In this reaction, Oxygen (O2) in water is reduced to Hydroxyl Ions (OH-).
O2 + 2H2O + 4e- → 4OH-
Please use a larger screen
to view the diagrams
on this page.
Currently viewing
Page last updated: 3/4/25
