Currently viewing
Menu
Electrochemical Tests - Introduction
In aqueous systems (water media), corrosion is an electrochemical process. An electrochemical process involves the "movement of electrons" (current flow). As a result, Electrochemical Tests have traditionally been used to gain insight into the rate and form of degradation.
Direct Current Electrochemical Tests
A typical Direct Current (DC) Electrochemical Corrosion Test would be carried out in a three electrode cell, as shown in the Figure below:
Direct Current Electrochemical Tests
A typical Direct Current (DC) Electrochemical Corrosion Test would be carried out in a three electrode cell, as shown in the Figure below:
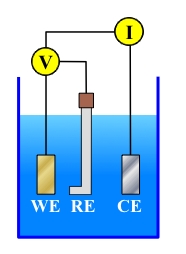
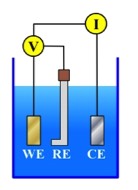
Working Electrode (WE)
Reference Electrode (RE)
Counter Electrode (CE)
The Working Electrode (WE) is the electrode the researcher is interested in assessing.
The Counter Electrode (CE) is the electrode that supports the corresponding reaction. During a DC electrochemical experiment, the potential and current of the WE change. For example, if the WE is polarized in the positive direction - such that the anodic process increases - the CE is polarized in the cathodic direction to support the increased cathodic reaction at the surface. Counter electrodes are generally fabricated from some relatively inert material - frequently platinum or graphite.
The Reference Electrode (RE) is an electrode with a stable known potential. The RE is used because it is not possible to measure the "absolute potential" of only one electrode. As a result, the "relative potential" is used. This is the potential of the WE "relative" to the RE. During a DC electrochemical test, the potential between the WE and the RE is measured. The current between the WE and the CE is measured at the same time.
Electrochemical Cell Design
The most basic step in performing an electrochemical test is the design of the cell. Although this is a basic step, it is very important. A poorly designed cell will produce inferior results, potentially leading to misinterpretation of the data. In general, the RE and the CE present few problems. However, it is critical that the WE design prevents waterline and crevice effects that can cause spurious data.
Current Density
Current Density (i) is an important concept in electrochemistry because it is directly related to the Corrosion Rate. In addition, Current Density makes it possible to compare the electrochemical response of samples having different areas.
The Counter Electrode (CE) is the electrode that supports the corresponding reaction. During a DC electrochemical experiment, the potential and current of the WE change. For example, if the WE is polarized in the positive direction - such that the anodic process increases - the CE is polarized in the cathodic direction to support the increased cathodic reaction at the surface. Counter electrodes are generally fabricated from some relatively inert material - frequently platinum or graphite.
The Reference Electrode (RE) is an electrode with a stable known potential. The RE is used because it is not possible to measure the "absolute potential" of only one electrode. As a result, the "relative potential" is used. This is the potential of the WE "relative" to the RE. During a DC electrochemical test, the potential between the WE and the RE is measured. The current between the WE and the CE is measured at the same time.
Electrochemical Cell Design
The most basic step in performing an electrochemical test is the design of the cell. Although this is a basic step, it is very important. A poorly designed cell will produce inferior results, potentially leading to misinterpretation of the data. In general, the RE and the CE present few problems. However, it is critical that the WE design prevents waterline and crevice effects that can cause spurious data.
Current Density
Current Density (i) is an important concept in electrochemistry because it is directly related to the Corrosion Rate. In addition, Current Density makes it possible to compare the electrochemical response of samples having different areas.
The Current Density is found by dividing the Current (I) by the Area (A) of the sample: i = I / A
The Current Density is found by dividing the Current (I) by the Area (A) of the sample:
i = I / A
Currently viewing
Page last updated: 3/4/25
