Currently viewing
Menu
Potentiodynamic Test
This Direct Current (DC) Test provides information on the behavior of a metal or alloy over a wide range of potentials.
The general flow of a typical Potentiodynamic Test is presented in the Figure below. Starting at the Ecorr (Corrosion Potential) or slightly cathodic to Ecorr, the potential is scanned in the positive (anodic) direction while recording the current. Depending on the test requirements, the potential scan may be reversed at some point.
This type of Test would generate a Potentiodynamic Curve
similar to the one in the Figure below.
This type of Test would generate a Potentiodynamic Curve
similar to the one in the Figure below.
This type of Test would generate a
Potentiodynamic Curve
similar to the one in the Figure below.
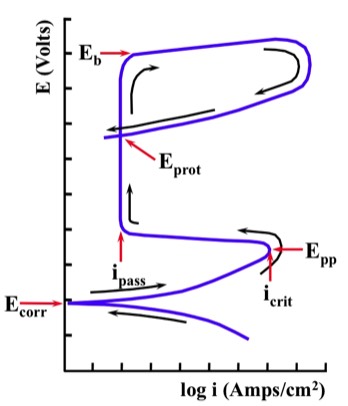
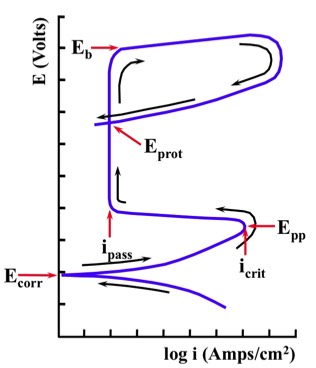
Many of the following "key values" may be defined during a Potentiodynamic Test.
"Ecorr" (Corrosion Potential) indicates the intersection of the anodic and cathodic curves.
If the sample is an active-passive alloy, at some point anodic to "Ecorr" a nose (the current density maximum) - followed by a significant decrease in current density - will be observed. The point where this occurs is defined by the "Epp" (Primary Passivation Potential) and the "icrit" (Critical Current Density for Passivation). These two "key values" (Epp and icrit) provide information about the ease with which a metal transitions from the active to the passive state.
As we continue to increase the voltage in the anodic direction, it will reach a potential region that exhibits a relatively low current density. This region may extend for many millivolts, and is due to the formation of a passive film on the surface of the alloy (for active-passive alloys). The region is defined by "ipass" (Passive Current Density), which can be used to obtain information on the corrosion rate of the metal when it is in the passive state.
As we continue to polarize in the anodic direction, we will eventually observe a substantial and rapid increase in current density. This increase can be associated with the onset of localized corrosion. It has been given various names, including "Eb" (Breakdown Potential) or "Epit" (Pitting Potential). This "key value" can be used to provide information on the probability that localized corrosion will develop.
After exceeding "Eb", the scan can be reversed so that the potential is moving in the cathodic direction. In this case, we may observe a loop in the E-log i curve (hysteresis).
The point where the forward and reverse scans intersect is termed "Eprot" (Protection Potential). Localized corrosion will not initiate at potentials below this point. If localized corrosion has already initiated, it will propagate at potentials between "Eprot" and "Eb". Localized corrosion can initiate at potentials above "Eb".
"Ecorr" (Corrosion Potential) indicates the intersection of the anodic and cathodic curves.
If the sample is an active-passive alloy, at some point anodic to "Ecorr" a nose (the current density maximum) - followed by a significant decrease in current density - will be observed. The point where this occurs is defined by the "Epp" (Primary Passivation Potential) and the "icrit" (Critical Current Density for Passivation). These two "key values" (Epp and icrit) provide information about the ease with which a metal transitions from the active to the passive state.
As we continue to increase the voltage in the anodic direction, it will reach a potential region that exhibits a relatively low current density. This region may extend for many millivolts, and is due to the formation of a passive film on the surface of the alloy (for active-passive alloys). The region is defined by "ipass" (Passive Current Density), which can be used to obtain information on the corrosion rate of the metal when it is in the passive state.
As we continue to polarize in the anodic direction, we will eventually observe a substantial and rapid increase in current density. This increase can be associated with the onset of localized corrosion. It has been given various names, including "Eb" (Breakdown Potential) or "Epit" (Pitting Potential). This "key value" can be used to provide information on the probability that localized corrosion will develop.
After exceeding "Eb", the scan can be reversed so that the potential is moving in the cathodic direction. In this case, we may observe a loop in the E-log i curve (hysteresis).
The point where the forward and reverse scans intersect is termed "Eprot" (Protection Potential). Localized corrosion will not initiate at potentials below this point. If localized corrosion has already initiated, it will propagate at potentials between "Eprot" and "Eb". Localized corrosion can initiate at potentials above "Eb".
Currently viewing
Page last updated: 3/4/25
