Currently viewing
Menu
Unit 10 - Galvanic Series

Schematic Representation
of Galvanic Corrosion
The Galvanic Series - a listing of selected metals and alloys in a specific environment can be used to help avoid problematic galvanic couples.
If two dissimilar metals are joined in an environment which results in corrosion, one of the metals will corrode at an accelerated rate and the other will corrode at a reduced rate. If two metals are close to each other in the Galvanic Series, corrosion is less likely to be severe.
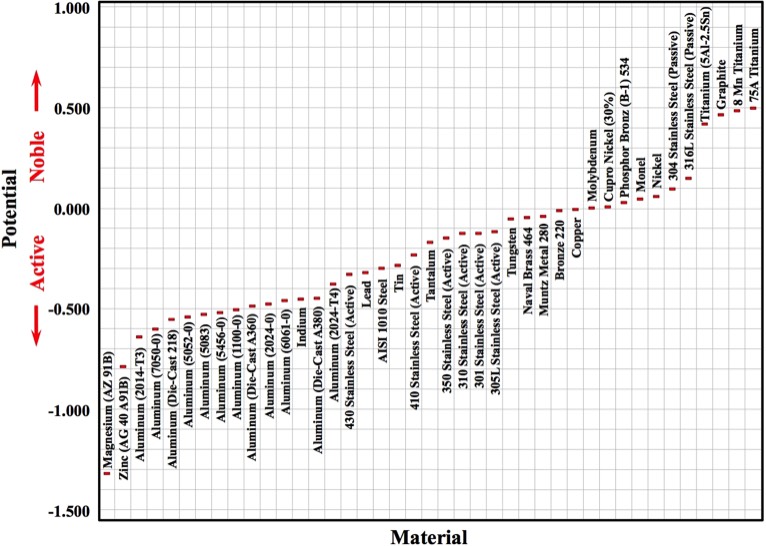
The Graph shown below was developed using selected data from Report No. RS-TR-67-11 (the U.S. Army Missile Command - 1967). In this Report, the authors coupled a 110 copper alloy (as a reference) to each of the metals and alloys being tested. Each metal couple was partially immersed in a 5% NaCl solution, and the potentials monitored.
The potential for each metal or alloy is represented by a red rectangle in the following Figure:
If two dissimilar metals are joined in an environment which results in corrosion, one of the metals will corrode at an accelerated rate and the other will corrode at a reduced rate. If two metals are close to each other in the Galvanic Series, corrosion is less likely to be severe.
The Graph shown below was developed using selected data from Report No. RS-TR-67-11 (the U.S. Army Missile Command - 1967). In this Report, the authors coupled a 110 copper alloy (as a reference) to each of the metals and alloys being tested. Each metal couple was partially immersed in a 5% NaCl solution, and the potentials monitored.
The potential for each metal or alloy is represented by a red rectangle in the following Figure:
Galvanic Corrosion may also be termed Bimetallic Corrosion.
The Galvanic Series - a listing of selected metals and alloys in a specific environment - can be used to help avoid problematic couples.
If two dissimilar metals are joined in an environment which results in corrosion, one of the metals will corrode at an accelerated rate and the other will corrode at a reduced rate. If two metals are close to each other in the Galvanic Series, corrosion is less likely to be severe.
The Galvanic Series - a listing of selected metals and alloys in a specific environment - can be used to help avoid problematic couples.
If two dissimilar metals are joined in an environment which results in corrosion, one of the metals will corrode at an accelerated rate and the other will corrode at a reduced rate. If two metals are close to each other in the Galvanic Series, corrosion is less likely to be severe.
Please use a larger screen
to view the
Galvanic Series Graph
on this page
Currently viewing
Page last updated: 3/4/25
