Currently viewing
Menu
Unit 9 - Kinetics of Corrosion
Kinetics can give us information on the rate of a corrosion reaction. Thermodynamics alone does not provide us with information on the rate of corrosion - even if a Pourbaix Diagram indicates that it can occur.
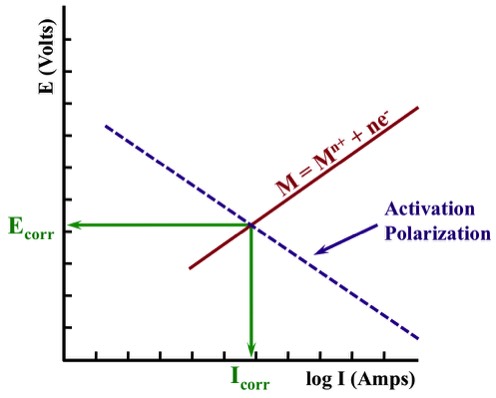
Activation Polarization
Activation Polarization involves a slow step in the reaction sequence, and is frequently associated with the Hydrogen Evolution Reaction (HER):
2H+ + 2e- ⇄ H2.
During the HER, hydrogen ions react with electrons to form Adsorbed Hydrogen (Hads). Adsorbed Hydrogen on the metal surface react to form a molecule - and molecules react to form hydrogen gas bubbles. Any of these steps could be the slow step.
Activation Polarization
Activation Polarization involves a slow step in the reaction sequence, and is frequently associated with the Hydrogen Evolution Reaction (HER):
2H+ + 2e- ⇄ H2.
During the HER, hydrogen ions react with electrons to form Adsorbed Hydrogen (Hads). Adsorbed Hydrogen on the metal surface react to form a molecule - and molecules react to form hydrogen gas bubbles. Any of these steps could be the slow step.
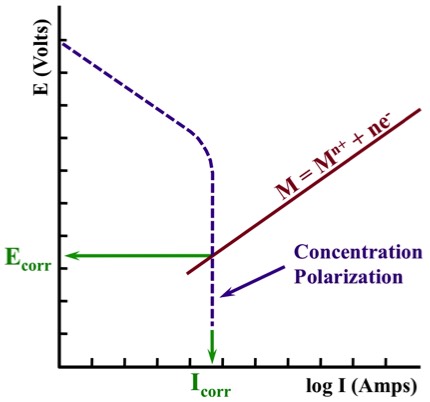
Concentration Polarization
During Concentration Polarization, the rate of the reaction is controlled by the diffusion (movement in solution) of a cathodic reactant. For corrosion, this is most frequently associated with the Oxygen Reduction Reaction (ORR):
2H2O + O2 + 4e- ⇄ 4OH-
Alternatively, the rate of the reaction can also be limited by the diffusion of an anodic reactant away from the surface; however, this is less common.
Currently viewing
Page last updated: 3/4/25
