Currently viewing
Menu
Stress Corrosion Cracking (SCC)
Stress Corrosion Cracking - cracking of a material produced by the combined action of corrosion and sustained tensile stress (residual or applied). [1]
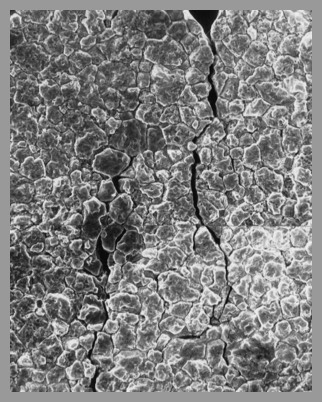
Stress Corrosion Cracking (SCC)
at the top of a
Type 316-L Stainless Steel U-Bend Sample
after exposure to a
highly chlorinated SCWO environment
Stress Corrosion Cracking (SCC)
at the top of a Type 316-L
Stainless Steel U-Bend Sample
after exposure to a
highly chlorinated SCWO environment
Figure: D.B. Mitton et al:
"Evaluating Stress Corrosion
and Corrosion Aspects in
Supercritical Water Oxidation Systems
for the Destruction of Hazardous Waste"
Paper 203, Corrosion '97 (1997).
© NACE International
For Stress Corrosion Cracking (SCC) to develop in an alloy, both static tensile stress and specific environmental factors must be present. Brass, for example, tends to exhibit SCC in "ammoniacal" (ammonia-containing) environments - while austenitic stainless steels are prone to cracking in aqueous chloride solutions.
SCC can still develop in an environment that would not normally result in cracking. This can happen if evaporation, boiling, or some other phenomenon concentrates an environment in such a way that cracking unexpectedly develops. If no apparent tensile stress is present, residual or other stresses from fabrication may be sufficient to cause SCC - even at stresses below the design stress.
It is important to remember that there can be substantial differences between the bulk environment and the local environment, and that it is the local environment which is significant.
Although the number of environment-alloy combinations that can result in SCC change with time, there are a number of well-known combinations such as: Carbon Steels exposed to caustic sodium hydroxide solutions AND Nickel-Chromium-Iron Alloys exposed to chloride solutions at elevated temperatures.
Stress corrosion cracks tend to be branched - with sharp tips. There is usually no corrosion product in the crack.
A number of mechanisms have been suggested for SCC. These include those relating to the rupture or cleavage of a surface film, and those that suggest adsorption of hydrogen or some critical anion. Each of the mechanisms proposed have their proponents and opponents.
SCC can still develop in an environment that would not normally result in cracking. This can happen if evaporation, boiling, or some other phenomenon concentrates an environment in such a way that cracking unexpectedly develops. If no apparent tensile stress is present, residual or other stresses from fabrication may be sufficient to cause SCC - even at stresses below the design stress.
It is important to remember that there can be substantial differences between the bulk environment and the local environment, and that it is the local environment which is significant.
Although the number of environment-alloy combinations that can result in SCC change with time, there are a number of well-known combinations such as: Carbon Steels exposed to caustic sodium hydroxide solutions AND Nickel-Chromium-Iron Alloys exposed to chloride solutions at elevated temperatures.
Stress corrosion cracks tend to be branched - with sharp tips. There is usually no corrosion product in the crack.
A number of mechanisms have been suggested for SCC. These include those relating to the rupture or cleavage of a surface film, and those that suggest adsorption of hydrogen or some critical anion. Each of the mechanisms proposed have their proponents and opponents.
[1] NACE/ASTM G193-10b Standard Terminology and Acronyms Relating to Corrosion, 2010. All rights reserved by NACE. (Reprinted with Permission)
Currently viewing
Page last updated: 3/4/25
