Currently viewing
Menu
Alternating Current (AC) Tests
Similar to a DC test, the Corrosion Rate or Penetration Rate can be found if you can define the Corrosion Current Density (icorr).
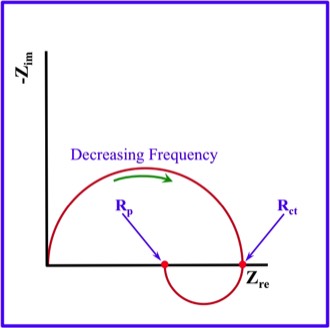
As presented in the Schematic Nyquist Plot in the Figure below, the value that will be obtained during EIS (Electrochemical Impedance Spectroscopy) will either be the Rct (Charge Transfer Resistance) or the Rp (Polarization Resistance).
As presented in the Schematic Nyquist Plot in the Figure below, the value that will be obtained during EIS (Electrochemical Impedance Spectroscopy) will either be the Rct (Charge Transfer Resistance) or the Rp (Polarization Resistance).
From the Stern and Geary relationship, the Corrosion Current (Icorr) in the next equation can be calculated if the Anodic (βa) and Cathodic (βc) Tafel Constants have been found previously, and the Polarization Resistance (Rp) is found during the current experiment. In the simplest case, the value for the Charge Transfer Resistance (Rct) is the same as for the Polarization Resistance (Rp). Even in more complex situations, using the value for Charge Transfer Resistance (Rct) may provide an estimate of the Corrosion Rate.
Icorr = βaβc/2.3(βa + βc)Rp
In order to determine the Corrosion Rate, we need to find the Corrosion Current Density (icorr). Most electrochemical software will do this automatically if you enter the Area of the sample. The icorr is simply the Corrosion Current (Icorr) per Unit Area (A), as follows:
icorr = Icorr/A
Once the icorr is known, it is a simple matter to find the corrosion rate (CR). In the following equation we already know the Atomic Mass (a), the Valence Change (n), and Faraday's Constant (F).
CR = icorra/nF
A Penetration Rate (such as mmpy or mpy) can be found by dividing by ρ (density).
Penetration Rate = icorra/nFρ
Currently viewing
Page last updated: 12/16/23
